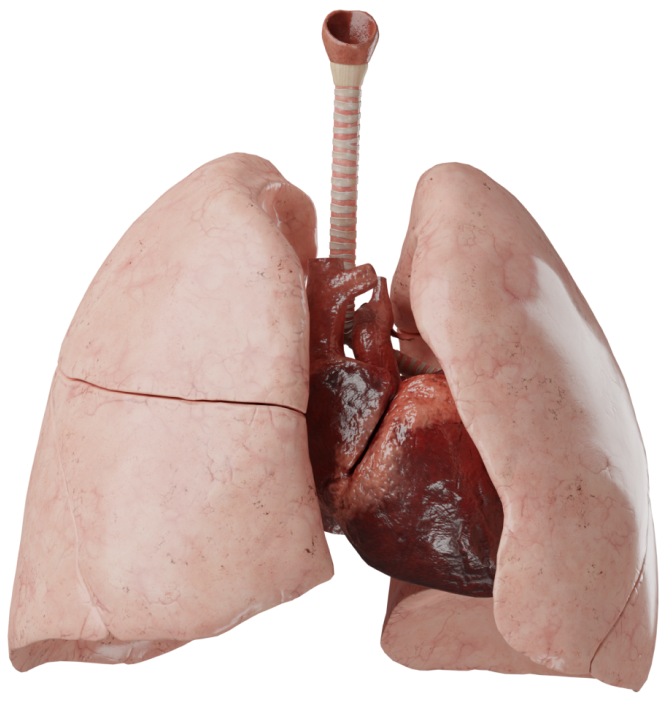
Атрезия легочной артерии с дефектом межжелудочковой перегородки
Что такое Атрезия легочной артерии с дефектом межжелудочковой перегородки?
Это сложный порок сердца, при котором определяется нарушение развития легочных артерий в широком диапазоне - от атрезии клапанов до полного их отсутствия. Также внутрисердечная анатомия схожа с тетрадой Фалло: ДМЖП, декстрапозиция аорты, гипертрофия правого желудочка. Именно поэтому многие относят АЛА с ДМЖП к группе ТФ с атрезией легочной артерии.
Эмбриология
АЛА в эмбрионально-анатомическом отношении принадлежит к порокам конотрункуса. Конотрункус включает в себя понятие эмбрионального ствола (truncus) - общего сосуда, который является выносящим трактом сердца 2-3-недельного эмбриона, и части сердца, соединяющей желудочки с артериальным стволом (conus arteriosus). Легочный ствол образуется в результате разделения трункуса и аортального мешка артериальной перегородкой с образованием аорты и легочного ствола.
Эмбриональный артериальный ствол делится на левую и правую вентральные аорты, которые соединяются, образуя дорсальную (нисходящую) аорту. От артериального ствола отходит 6 пар аортальных дуг. В норме I–V пары запустевают. Из вентральной части VI пары формируются правая и левая легочные артерии.
В образовании легочного артериального дерева участвуют три самостоятельных анатомических сегмента. Из эмбрионального ствола при делении его артериальной перегородкой формируются аорта и легочной ствол, из правой и левой пары аортальных дуг образуются легочные артерии, из легочного сосудистого сплетения, которое непосредственно связано с дорсальной аортой, формируются внутрилегочные сосуды. Начиная со 2-го месяца эмбрионального развития формируется и дифференцируется система легочных артерий.
Врастание эндотелиальных отростков легочных артерий в зачаток легкого и контакт их с первичными сосудистыми образованиями индуцирует органный ангиогенез. Это проявляется в увеличении количества сосудистых формаций и развитии кровеносной системы легкого.
В результате нарушения нормального развития легочной артериальной системы происходит задержка роста и перерождение в фиброзную ткань определенных элементов системы легочной артерии с образованием атрезии. В частности, при нарушении развития VI пары аортальных дуг могут полностью отсутствовать одна или обе центральные легочные артерии. При этих формах порока лёгочное сосудистое сплетение кровоснабжается персистирующими примитивными межсегментарными артериями, берущими начало от нисходящей грудной аорты и не исчезнувшими вследствие инволюционных процессов.
Анатомия
Данный порок может быть как самостоятельным, так и сочетаться с другими пороками, чаще всего из группы одножелудочковых пороков. Характерными признаками АЛА с ДМЖП являются:
- атрезия легочной артерии на разных уровнях;
- ДМЖП;
- наличие коллатеральных сосудов;
- гипертрофия правого желудочка;
- декстрапозиция аорты.
Классификация
Анатомия легочных артерий отличается полиморфизмом, исчерпывающая классификация легочной артериальной патологии отсутствует, в хирургической практике преобладает индивидуальная характеристика порока, которая носит описательный характер.
Среди множества предложенных классификаций наиболее удобной для клинического применения и наиболее распространенной является классификация Somerville, согласно которой выделяется четыре типа порока:
- I - атрезия на клапанном уровне или на уровне ВОПЖ;
- II - ствол отсутствует, дуктус-зависимое кровоснабжение легких;
- III - атрезия/гипоплазия правой (IIIa) и левой (IIIb) легочных артерий, ствола. Присутствуют аортолегочные коллатерали;
- IV - легочные артерии отсутствуют. Обязательно присутствуют коллатеральные артерии.
Гемодинамика
При данном пороке легкие и правый желудочек полностью разобщены, поэтому венозная кровь не попадает в легкие антеградно. Выживание пациентов полностью зависит от наличия и функции альтернативных источников легочного кровоснабжения. Благодаря наличию большого ДМЖП обеспечивается шунтирование крови в левый желудочек, а затем в аорту. Правый желудочек работает в условиях системного давления, что приводит к его гипертрофии. Смешанная кровь поступает в аорту, затем через артериальный проток в коллатеральные сосуды и в легкие. В результате миксинга крови, насыщение ее кислородом снижено и все органы находятся в состоянии хронической гипоксии.
В легких могут одновременно существовать сегменты, которые обеспечиваются только истинными легочными артериями или только дискретными аортолегочными коллатералями в изолированных бронхолегочных сегментах, и сегментах с двойным кровоснабжением.
Гипертензия и стенозы внутрилегочных артерий создают мозаичную картину кровоснабжения отдельных долей и сегментов, в результате чего некоторые из них могут находиться в состоянии легочной гипертензии с тяжелыми склеротическими поражениями сосудов, тогда как в других участках легких такие изменения не наблюдаются.
Угроза развития стойкой легочной гипертензии существует уже после периода новорожденности. Стенозы коллатеральных артерий создают турбулентность тока крови, которая в сочетании с полицитемией и повышением вязкости крови способствует увеличению обструкции коллатерали, в некоторых случаях вплоть до полного функционального закрытия.
Диагностика
- ЭхоКГ. Возможно обнаружить порок, оценить размеры ДМЖП, расположение магистральных сосудов, размеры камер сердца.
- ЭКГ. Изменения неспецифичны. Возможно обнаружить признаки гипертрофии правого желудочка.
- Рентгенография органов грудной клетки. Признаки обеднения легочного кровотока.
- Катетеризация сердца. Необходима для определения коллатеральных сосудов, их размера, количества и оценки гемодинамических функций.
Клинические проявления
АЛА-ДМЖП является цианотичным пороком (кожные покровы и видимые слизистые цианотичны). Интенсивность цианоза зависит от характера и степени развития коллатерального кровообращения. Если коллатерали создают нормальное или избыточное легочное кровообращение, системная гипоксемия может долгое время находиться в состоянии субкомпенсации. Это обеспечивает на определенное время удовлетворительное физическое развитие. Но даже при избыточном легочном кровотоке цианоз возникает в результате смешивания артериальной и венозной крови. Если коллатерали создают нормальное или избыточное легочное кровообращение, системная гипоксемия может долгое время находиться в состоянии субкомпенсации.
Это обеспечивает, на определенное время, удовлетворительное физическое развитие. Серьезное влияние на естественное течение оказывают гипертензивные коллатеральные артерии, которые находятся под системным артериальным давлением. Это приводит к развитию обструктивных изменений в артериолах соответствующих участков легочной ткани.
Полное отсутствие выброса крови из правого желудочка в легочную артерию делает этот порок «немым», однако в большинстве случаев над сердцем выслушиваются нехарактерные систолические шумы разной интенсивности или непрерывный систоло-диастолический шум коллатералей. У маленьких детей на клинические проявления аномалии влияют отличия двух главных источников легочного кровоснабжения — ОАП и коллатеральные артерии.
Лечение
Большинство детей первого года жизни с АЛА-ДМЖП в связи с тяжелым состоянием нуждаются в интенсивной терапии.
Пролонгированное внутривенное введение простагландина недоношенным детям с малой массой и недоразвитым для своего возраста младенцам улучшает артериальную оксигенацию и обеспечивает клиническое улучшение. Необходимо иметь в виду, что у некоторых младенцев со сложными формами АЛА может не быть врожденного ОАП, а может быть увеличенный легочный кровоток и сердечная недостаточность, требующая адекватного медикаментозного лечения. Радикальная коррекция может быть выполнена как первичная операция при благоприятной анатомии легочной артерии, обычно на первом году жизни больного, или в качестве второго этапа коррекции после паллиативного вмешательства. Цель паллиативного лечения заключается в увеличении объема легочного кровотока и подготовке к радикальной коррекции порока.
При АЛА с ДМЖП рекомендуется выполнение следующих типов паллиативных операций: создание системно-легочных анастомозов, реконструкция путей оттока ПЖ без пластики ДМЖП, стентирование ОАП, легочных артерий.
Целью этапного хирургического лечения порока является пластика ДМЖП, создание адекватного сообщения между правым желудочком и системой ЛА, ликвидация внесердечных источников кровотока в легких, восстановление истинного легочного кровотока с использованием методов унифокализации. Первым этапом иногда выполняется операция реконструкции путей оттока правого желудочка без пластики ДМЖП. При I типе иссекаются все компоненты многоуровневого стеноза, выполняется пластика выводного тракта ПЖ и ствола ЛА заплатой. При других типах порока имплантируется искусственный ствол ЛА. При наличии большого количества коллатеральных артерий и недостаточном развитии истинных легочныйх артерий выполняется операция унифокализации легочных артерий. Из всех коллатеральных и истинных артерий формируется единая сеть, которая затем анастомозируется с правым желудочком с помощью синтетического протеза.